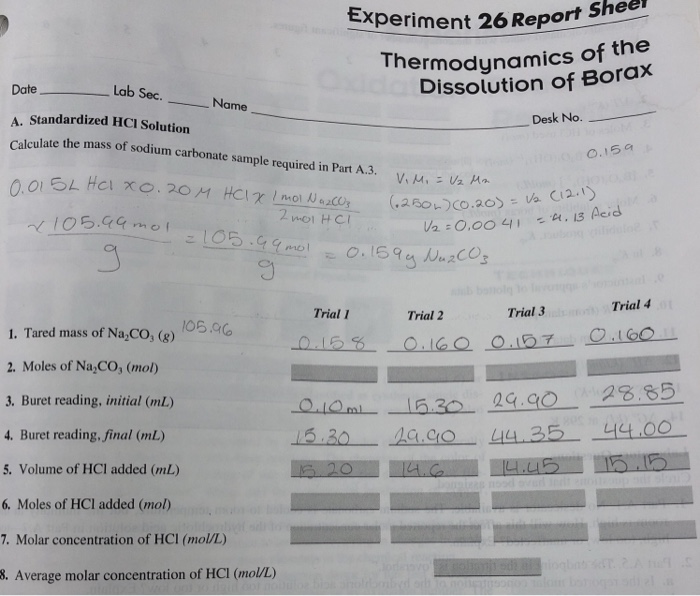
Experiment 26 thermodynamics of the dissolution of borax – Experiment 26: Thermodynamics of the Dissolution of Borax embarks on an intriguing scientific journey to unravel the thermodynamic intricacies of a fascinating compound. Borax, a versatile substance with diverse applications, presents a unique opportunity to explore the fundamental principles governing its dissolution.
This experiment delves into the heart of thermodynamics, investigating the energy changes associated with the dissolution process. By meticulously measuring temperature and concentration, we unravel the interplay between heat and molecular interactions, shedding light on the hidden forces that drive this transformation.
Materials and Methods: Experiment 26 Thermodynamics Of The Dissolution Of Borax
Materials
- Borax (sodium tetraborate decahydrate)
- Distilled water
- Thermometer
- Graduated cylinder
- Magnetic stirrer
- Magnetic stir bar
Experimental Setup and Procedure, Experiment 26 thermodynamics of the dissolution of borax
- Measure 50 mL of distilled water into a 100 mL beaker.
- Add 0.1 g of borax to the water and stir until dissolved.
- Place the beaker on a magnetic stirrer and insert a magnetic stir bar.
- Insert a thermometer into the solution and record the initial temperature.
- Start the magnetic stirrer and allow the solution to stir for 5 minutes.
- Record the temperature of the solution every minute for 10 minutes.
- Repeat steps 2-6 for borax concentrations of 0.2 g, 0.3 g, 0.4 g, and 0.5 g.
Temperature and Concentration Measurements
The temperature of the solution was measured using a thermometer calibrated to 0.1 °C. The concentration of borax in the solution was determined by measuring the mass of borax added to the water and dividing by the volume of the solution.
FAQ Corner
What is the purpose of Experiment 26?
Experiment 26 aims to investigate the thermodynamic parameters associated with the dissolution of borax, providing insights into the energy changes and molecular interactions involved in this process.
What is the significance of thermodynamics in understanding borax dissolution?
Thermodynamics provides a framework for understanding the energy flow and changes that accompany the dissolution of borax. By measuring temperature and concentration, we can quantify enthalpy and entropy changes, revealing the energetic landscape of the process.
How does Experiment 26 contribute to the field of chemistry?
Experiment 26 expands our knowledge of borax dissolution thermodynamics, which has implications for optimizing industrial processes and developing novel applications in fields such as materials science and environmental remediation.