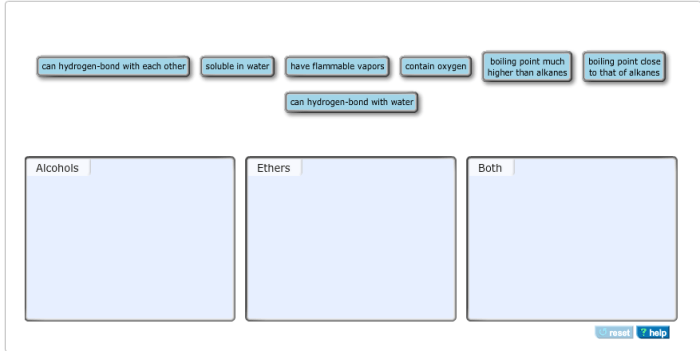
Identify each property as that of alcohols ethers or both – Alcohols and ethers are two classes of organic compounds that share some similarities but also have distinct properties. This article aims to identify and explain the characteristic properties of alcohols and ethers, including their physical and chemical properties, nomenclature, spectroscopy, and applications.
The contrasting properties of alcohols and ethers stem from the differences in their molecular structures. Alcohols possess a hydroxyl group (-OH), while ethers have an ether linkage (-O-). These structural variations lead to distinct physical and chemical behaviors.
Physical Properties
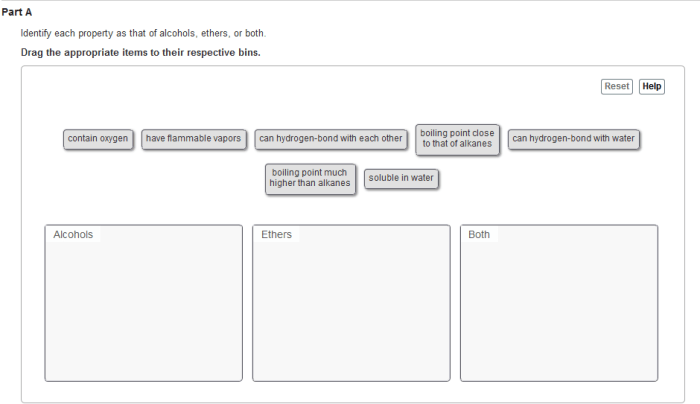
Alcohols and ethers are both organic compounds that contain oxygen. However, they differ in their physical properties due to differences in their molecular structures.
Alcohols have a hydroxyl group (-OH) attached to a carbon atom, while ethers have an oxygen atom bonded to two carbon atoms. This difference in structure leads to differences in their boiling points, densities, and solubilities in water.
Boiling Points
Alcohols have higher boiling points than ethers of comparable molecular weight. This is because alcohols can form hydrogen bonds with each other, which increases the intermolecular forces between them. Ethers, on the other hand, cannot form hydrogen bonds with each other, so their intermolecular forces are weaker.
Densities
Alcohols are denser than ethers of comparable molecular weight. This is because the hydroxyl group in alcohols is more polar than the oxygen atom in ethers. The polar hydroxyl group interacts more strongly with other molecules, which increases the density of the liquid.
Solubilities in Water, Identify each property as that of alcohols ethers or both
Alcohols are more soluble in water than ethers of comparable molecular weight. This is because the hydroxyl group in alcohols can form hydrogen bonds with water molecules. Ethers, on the other hand, cannot form hydrogen bonds with water molecules, so they are less soluble in water.
User Queries: Identify Each Property As That Of Alcohols Ethers Or Both
What is the key difference between alcohols and ethers?
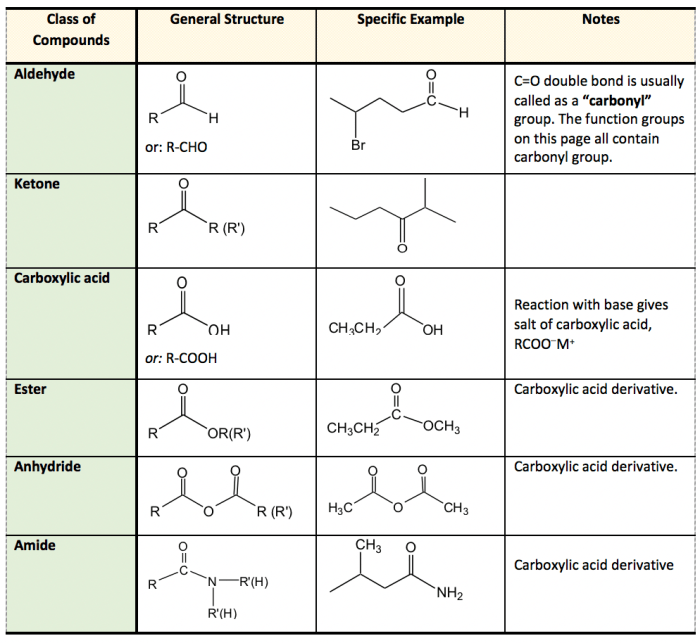
The key difference lies in their functional groups: alcohols have a hydroxyl group (-OH), while ethers have an ether linkage (-O-).
How do the physical properties of alcohols and ethers differ?
Alcohols generally have higher boiling points and are more soluble in water than ethers due to the presence of the hydroxyl group, which forms hydrogen bonds.
What are some characteristic chemical reactions of alcohols and ethers?
Alcohols undergo reactions with acids, bases, and oxidizing agents, while ethers are relatively inert and undergo limited reactions.