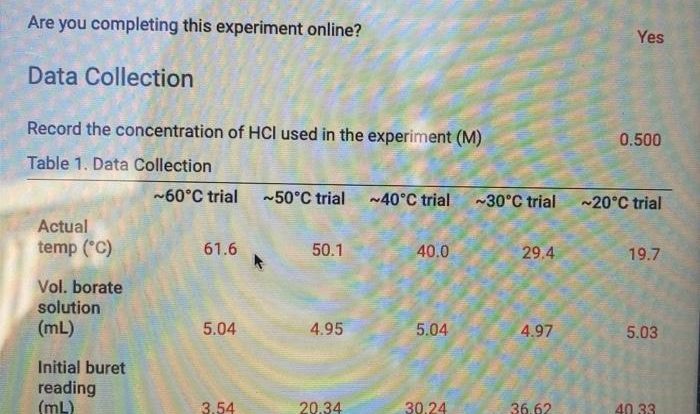
Chemistry and chemical reactivity 10th edition – Chemistry and Chemical Reactivity, 10th Edition, unveils the captivating world of chemical principles and reactions, inviting readers to explore the fundamental building blocks of matter and the dynamic forces that drive its transformations. This comprehensive textbook empowers students with a thorough understanding of the intricate relationships between structure, bonding, and reactivity, equipping them to navigate the complexities of chemical phenomena.
Delving into the core concepts of chemistry, this edition provides a comprehensive examination of atomic structure, bonding theories, and intermolecular interactions. It unravels the mysteries of chemical reactions, showcasing their applications across diverse fields, from medicine to materials science. The text meticulously explores the laws of thermodynamics and chemical equilibrium, providing a framework for predicting reaction outcomes and understanding the driving forces behind chemical processes.
1. Key Concepts in Chemistry and Chemical Reactivity
Chemistry is the study of matter and its interactions. It encompasses the fundamental principles of atomic structure, bonding, and intermolecular forces. Chemical reactions are processes that transform one set of substances into another, and they play a crucial role in various fields, including medicine, materials science, and energy.
Atomic Structure
- Atoms consist of a nucleus, which contains protons and neutrons, and electrons that orbit the nucleus.
- The number of protons in an atom determines its element, while the number of electrons determines its chemical properties.
- The arrangement of electrons in orbitals governs the atom’s size, shape, and reactivity.
Bonding
- Chemical bonds are forces that hold atoms together to form molecules and compounds.
- There are various types of bonds, including covalent bonds, ionic bonds, and metallic bonds.
- The type of bond formed depends on the electronegativity and size of the atoms involved.
Intermolecular Forces
- Intermolecular forces are weaker than chemical bonds, but they play a significant role in determining the physical properties of substances.
- Types of intermolecular forces include dipole-dipole interactions, hydrogen bonding, and van der Waals forces.
- These forces influence properties such as melting point, boiling point, and solubility.
2. Thermodynamics and Chemical Equilibrium

Thermodynamics is the study of energy and its transformations. Chemical equilibrium is a state in which the forward and reverse reactions of a chemical reaction occur at the same rate, resulting in no net change in the concentrations of the reactants and products.
Laws of Thermodynamics
- The first law of thermodynamics states that energy cannot be created or destroyed, only transferred or transformed.
- The second law of thermodynamics states that the entropy of an isolated system always increases over time.
- The third law of thermodynamics states that the entropy of a perfect crystal at absolute zero is zero.
Chemical Equilibrium
- Chemical equilibrium is a dynamic process where the forward and reverse reactions occur simultaneously.
- The equilibrium constant is a value that describes the relative amounts of reactants and products at equilibrium.
- Factors that affect equilibrium include temperature, pressure, and the concentration of reactants and products.
3. Kinetics and Reaction Mechanisms
Kinetics is the study of the rates of chemical reactions. Reaction mechanisms are the detailed steps by which a chemical reaction occurs.
Factors Affecting Reaction Rates, Chemistry and chemical reactivity 10th edition
- Concentration of reactants
- Temperature
- Surface area of reactants
- Nature of the reactants
- Presence of a catalyst
Reaction Mechanisms
- Reaction mechanisms involve a series of elementary steps, each with its own rate constant.
- The overall rate of a reaction is determined by the slowest step, known as the rate-determining step.
- Reaction mechanisms can be determined using experimental techniques such as isotope labeling and spectroscopy.
Frequently Asked Questions: Chemistry And Chemical Reactivity 10th Edition
What are the key concepts covered in Chemistry and Chemical Reactivity, 10th Edition?
The textbook covers fundamental principles of chemistry, including atomic structure, bonding, intermolecular forces, chemical reactions, thermodynamics, chemical equilibrium, reaction kinetics, acids, bases, aqueous equilibria, electrochemistry, organic chemistry, inorganic chemistry, analytical chemistry, biochemistry, and environmental chemistry.
How does the textbook approach the study of chemical reactions?
The text provides a comprehensive examination of chemical reactions, exploring their mechanisms, applications, and the factors that influence their rates. It emphasizes the importance of understanding reaction pathways and energy changes to predict reaction outcomes.
What are the strengths of Chemistry and Chemical Reactivity, 10th Edition?
The textbook’s strengths lie in its clear and concise explanations, comprehensive coverage of topics, and integration of real-world examples. It provides a solid foundation in chemistry for students pursuing further studies or careers in science-related fields.