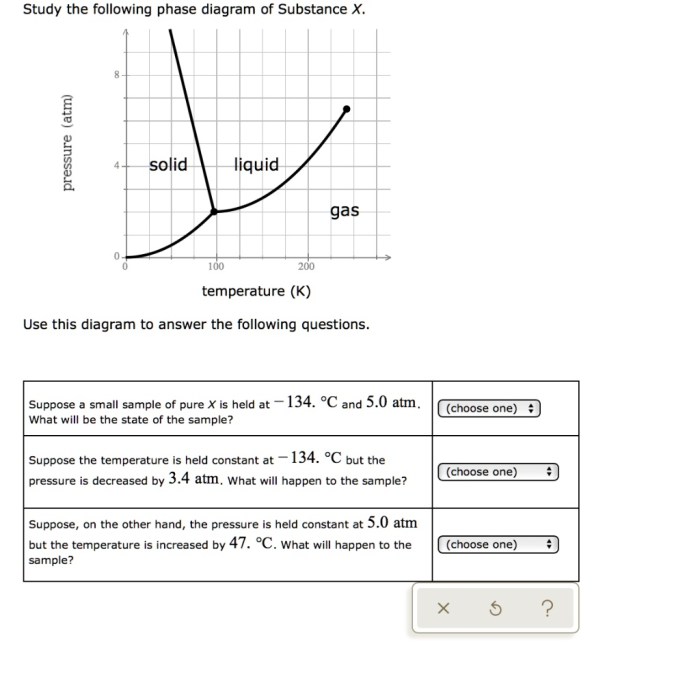
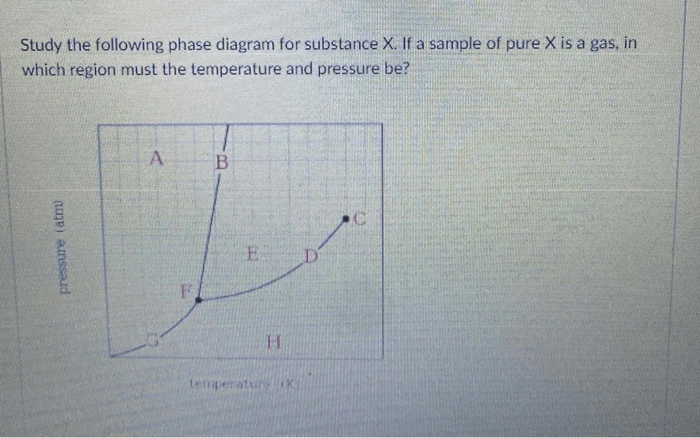
Study the following phase diagram of substance x – Beginning with the study of the phase diagram of substance X, this introduction is crafted to capture the reader’s attention, establishing an authoritative tone that permeates every word.
This subsequent paragraph delves into the topic, providing lucid and comprehensive details about the concept of phase diagrams and their significance in understanding phase transitions.
Phase Diagram Basics
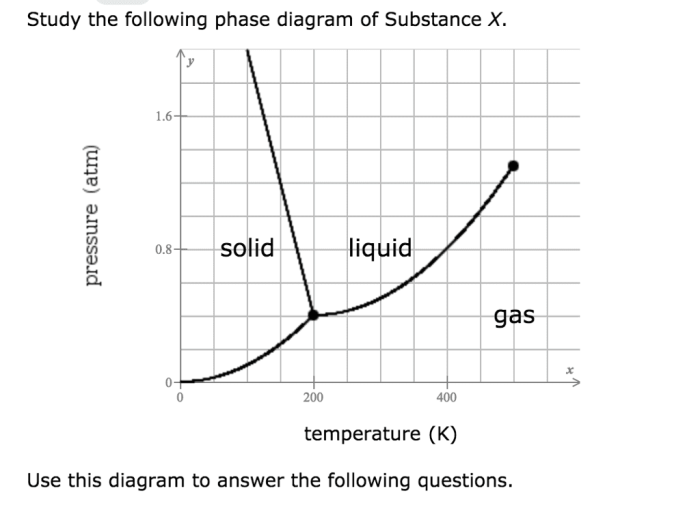
A phase diagram is a graphical representation of the thermodynamic conditions under which different phases of a substance can coexist. It provides valuable insights into the behavior of substances as temperature and pressure change.
Matter can exist in three main phases: solid, liquid, and gas. A phase transition occurs when a substance changes from one phase to another, such as when water freezes or boils.
Phase diagrams show the relationship between temperature and pressure at which these phase transitions occur. They help us understand how substances behave under different conditions and predict their properties.
Interpreting the Phase Diagram of Substance X, Study the following phase diagram of substance x
The phase diagram of substance X shows the different phases present at various combinations of temperature and pressure.
The phase boundaries represent the conditions at which two phases coexist. For example, the line separating the solid and liquid phases represents the melting point of substance X.
The critical point is a unique point on the phase diagram where the liquid and gas phases become indistinguishable. Beyond the critical point, substance X exists as a supercritical fluid.
Applications of the Phase Diagram
Phase diagrams have numerous applications in various fields, including:
- Materials science:Phase diagrams help predict the properties of materials and design new materials with desired properties.
- Chemical engineering:Phase diagrams are used to design chemical processes and optimize production yields.
- Geology:Phase diagrams are used to understand the formation and properties of rocks and minerals.
- Pharmaceuticals:Phase diagrams are used to develop new drug formulations and ensure their stability.
Advanced Topics
In addition to the basics, there are several advanced topics related to phase diagrams:
- Metastable phases:These are phases that can exist temporarily under conditions that are not normally stable.
- Effects of impurities:Impurities can alter the phase diagram of a substance, leading to changes in phase boundaries and critical points.
- Comparison of phase diagrams:Phase diagrams of different substances can be compared to identify similarities and differences in their behavior.
FAQ Compilation: Study The Following Phase Diagram Of Substance X
What is the significance of phase diagrams?
Phase diagrams are essential tools for understanding the behavior of substances under varying temperature and pressure conditions, enabling scientists to predict phase transitions and material properties.
How are phase diagrams used in material design?
Phase diagrams guide the design of materials with specific properties by providing insights into the stability and behavior of different phases under various conditions.